Ever wondered how gin is made? Well, we have the process of distillation to thank for our favourite spirit – but how does it work? Let’s learn all about delightful distillation, the magical science that turns water, alcohol and botanicals into gin!
The history of distillation is long and illustrious – in fact, you could say it’s paved with gold! There’s evidence that the Babylonians had a rough grasp of the distillation process more than 3,000 years ago, and they didn’t keep it a secret. Ancient civilisations in China, Pakistan and Europe were distilling by the 1st century BC, including the ancient Romans. The science of distillation developed further thanks to alchemy. This discipline – somewhere between science and mysticism – was entirely concerned with turning materials into gold. While no alchemist has ever succeeded (as far as we know!) these obsessive tinkerers developed many of the mechanical
advances that enabled the distillation of spirits later on. The first book written about distillation was published by a German in 1500, but at that time the process was used to create perfumes or medicines. Only 100 years later did references to drinking spirits for fun start to gain traction. But, by the 19th century, distillation in its modern incarnation was born!
How Does Distillation Work?
Step One: Get Your Equipment
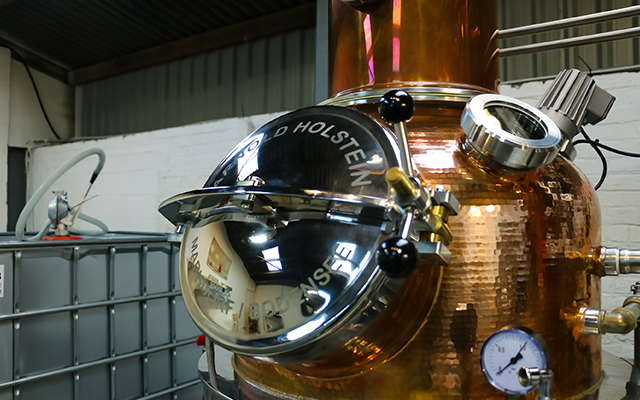
Distillers work on stills, which can be either pot stills (the oldest type of still) or column stills, complex pieces of machinery with columns stretching up into the ceiling. The still is connected to a condenser (a container through which cold water is run) which in turn is connected to a barrel to collect the finished distillate.
Step Two: Clean Your Still
Your still must be cleaned thoroughly before every distillation. After all, you wouldn’t want the grime from the last distillation to mess up your gin!
Step Three: Add Your Alcohol – And Botanicals!
There are many unique distillation techniques, often developed by distillers over months and years of experimentation. But the basics remain the same: you begin by adding the botanicals and your base spirit, also called a ‘neutral spirit’, to the body of the still. This spirit is a fermented alcohol, usually made of grain, with no additional flavour. It’s a combination of water and pure alcohol.
Step Four: Turn Up the Heat
A heat source – some distillers use open flames, while others use furnaces or electric heat sources – starts to heat the bottom of the still. Alcohol begins to boil at 73 degrees Celsius, while water won’t boil until it reaches 100 degrees. This means that the alcohol in the neutral spirit will become a gas and begin to rise while most of the water stays just where it is in the bottom of the still.
Step Five: Cool and Capture
As a gas, the alcohol moves up through the still head, or the top of the pot still, or the columns of the column still. It then moves through the condenser, which is filled with cold water, where it cools down and turns back into a liquid. The final distillate trickles into the barrel, where the distiller can decide whether to keep it or return it to the still for a second round of distillation, to become an even higher-proof solution of water and alcohol.













 plus all of your
plus all of your
 See Bronze Benefits
See Bronze Benefits
 plus all of your
plus all of your
 See silver Benefits
See silver Benefits
 plus all of your
plus all of your
 See gold Benefits
See gold Benefits